- 1571 – Investigational New Drug Application
- 1572 – Statement of Investigator
- 3674 - Certification of Compliance with Requirements of ClinicalTrials.gov Data Bank
- Protocol
- Summary of Previous Human Experience with the Investigational Drug
- Animal Pharmacology and Toxicology (PT)
*Often investigator-sponsored research INDs will address the requirements for nonclinical and CMC information by providing a Letter of Authorization (LOA) from the commercial manufacturer. The LOA allows FDA to reference the nonclinical and CMC information in the commercial manufacturer’s IND on behalf of the sponsor-investigator, to fulfill the requirements.
The key difference between the submission of commercial vs. research INDs is that commercial INDs must be submitted in electronic format, whereas electronic submission standards for research INDs are highly encouraged but optional.
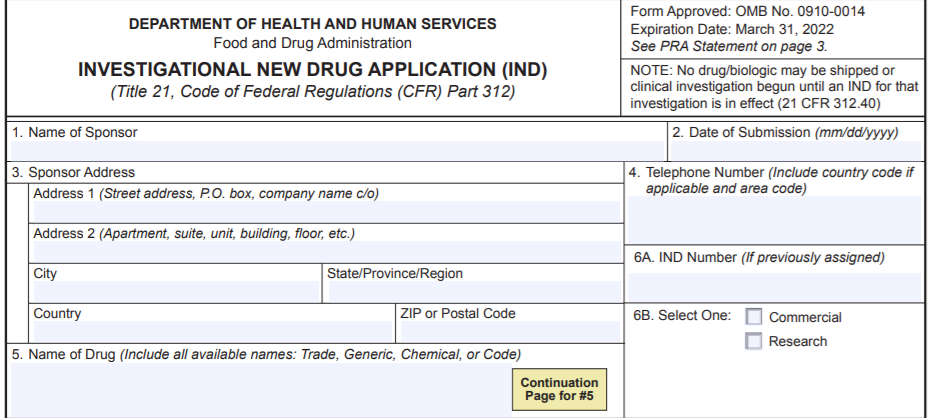
When a sponsor of a research IND submits either a Phase 2 or Phase 3 protocol, the IND will normally then be considered “commercial” and eCTD requirements would become applicable. In this case, the sponsor should select “Commercial” on FDA Form 1571 Field 6B: IND Type. However, if the product under investigation is not intended to be commercialized at a later date (i.e., the intent of the Phase 2 or Phase 3 protocol is still solely for research), the sponsor should submit a justification explaining their rationale in the cover letter, along with the protocol, and the sponsor should select “Research” on FDA Form 1571. If the FDA agrees, the IND will remain a “Research” IND and the eCTD requirements will not apply. Note that in all cases, expanded access INDs and protocols should be marked as “Research” on the Form 1571 and are exempt from eCTD requirements.
Research INDs in paper format should be mailed to the Central Document Room. Sponsors submitting in paper are expected to send their applications in triplicate (one original and two copies). FDA will notify the sponsor of the date it receives the application through an IND acknowledgment letter.
The IND application goes into effect 30 days after FDA receives the application, unless FDA notifies the sponsor that the investigations described in the application are subject to a clinical hold, or on earlier notification by FDA that the clinical investigations in the IND may begin. Once an IND application is in effect, a drug manufacturer may ship the investigational new drug to the investigator(s) named in the application. An investigator may not administer an investigational new drug to human subjects until the IND application goes into effect.
Additional information on submission of research INDs is located on our Investigator-Initiated Investigational New Drug (IND) Applications webpage.
Renu Lal, Pharm.D.
CDER Small Business and Industry Assistance
Issues of this newsletter are archived at http://www.fda.gov/cdersbiachronicles
This communication is consistent with 21CFR10.85(k) and constitutes an informal communication that represents our best judgment at this time but does not constitute an advisory opinion, does not necessarily represent the formal position of the FDA, and does not bind or otherwise obligate or commit the agency to the views expressed.